Compliance
The Sentry System Was Carefully Developed Under the GAMP4 Program to Ensure Proper Life-Cycle Documentation Required for a Validation Friendly Design
As an organization that must meet its own accreditation and compliance standards, Coldchain understands the process of validating critical systems and we have designed Sentry to assist your cGMP, FDA CFR part 11, the Joint Commision, USP797, HACCP, WHO/GDP or ISO17025 compliance program.
Vaccine for Children Program
Vulnerabilities In Vaccine Management
Department of Health and Human Services OFFICE OF THE INSPECTOR GENERAL Report on the Inspection and Evaluation of 45 VFC Providers and Results
Learn More
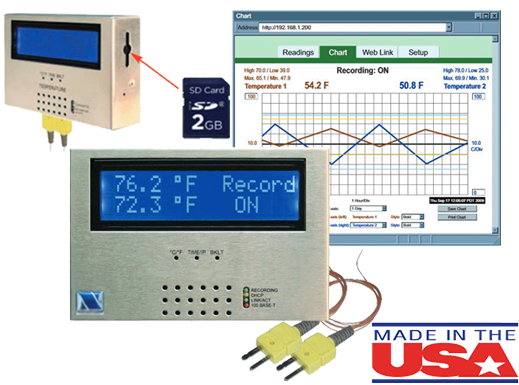
Data Acquisition Hardware
Our hardware is proudly made in the U.S.A. and provides a full featured local monitoring that continuously updates the Sentry Software Plaform with your critical data values. If the connection is interrupted, the device continues to display and log your data, reconnecting when the network connection is restored.
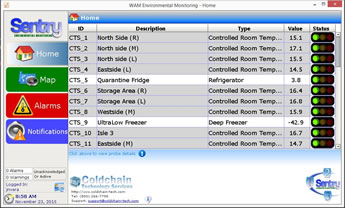
Sentry Monitoring
Asset Resource Terminal Interface
The SMART Interface give you full control and analysis capabilities wherever you are. There is no limit to the number of devices you can download the Sentry Shortcut onto. You can analyze trends, check on user alarm acknowledgements, activate alarms, change alarm parameters, and export/print reports or raw data values.